
Synthesis of Sodium Benzotriazole
- Synthesis from Benzene-1,2,4-triamin
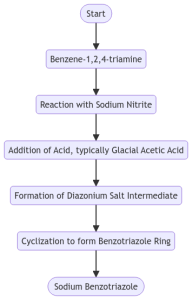
Figure 2. Synthesis from Benzene-1,2,4-triamine
One of the common routes for the synthesis of sodium benzotriazole involves the reaction of benzene-1,2,4-triamine with sodium nitrite in the presence of an acid, typically glacial acetic acid[1]. The reaction proceeds via the formation of a diazonium salt intermediate, which undergoes cyclization to form the benzotriazole ring.
- Synthesis from o-Nitroaniline Derivatives
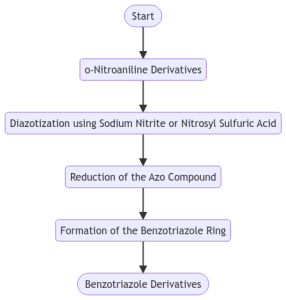
Figure 3. Synthesis from o-Nitroaniline Derivatives
Another approach involves the diazotization of alkyl-substituted o-nitroaniline derivatives using sodium nitrite or nitrosyl sulfuric acid, followed by reduction of the resulting azo compound to form the benzotriazole ring[2]. This method allows for the synthesis of benzotriazole derivatives with various substituents at the 5-position of the benzene ring.
- Synthesis from Sodium Azide and Arynes
A transition-metal-free synthesis of N-H and N-aryl benzotriazoles has been reported, involving the [3+2] annulation of sodium azide with arynes generated from 2-(trimethylsilyl)aryltriflates[3]. This method provides a selective route to different benzotriazole derivatives by varying the reaction conditions and fluoride sources.
- Synthesis from Benzotriazole Precursors
Several studies have explored the synthesis of sodium benzotriazole derivatives from benzotriazole precursors. For instance, the reaction of sodium phosphite with 1-(chloromethyl)benzotriazole has been used to synthesize 1-benzotriazolmethyl phosphonate[4]. Additionally, the reaction of benzotriazole with 2-bromoacetic acid in aqueous sodium hydroxide yields 2-(1H-benzo[d]triazol-1-yl)acetic acid, which can be further functionalized[5].
References
- Pochaiah, B., et al., Synthesis of Some Novel Benzotriazole-1-yl-acetic acid substituted aryl hydrazide Derivatives as Anticonvulsant Agents.Asian Journal of Research in Chemistry, 2013. 6: p. 71-75.
- 우드머빈게일, et al. Processes for the preparation of benzotriazole UV absorbers. 2001.
- Guin, A., et al., Selective Synthesis of N-H and N-Aryl Benzotriazoles by the [3 + 2] Annulation of Sodium Azide with Arynes.J Org Chem, 2019. 84(19): p. 12692-12699.
- Huang*, X. and H. Qian, SYNTHESIS OF 1-BENZOTRIAZOLMETHYL PHOSPHONATE BY REACTION OF SODIUM PHOSPHITE WITH 1-(CHLOROMETHYL)BENZOTRIAZOLE.Synthetic Communications, 1999. 29: p. 803-808.
- Gong, Y., et al., Synthesis of New Benzotriazole Derivatives Containing Carbon Chains as the Corrosion Inhibitors for Copper in Sodium Chloride Solution.Industrial & Engineering Chemistry Research, 2015. 54: p. 12242-12253.
