
3-Amino-5-Mercapto-1,2,4-Triazole

Specifications
| Item | Specification |
| Main content | ≥98.0﹪ |
| Appearance | white crystalline powder |
| Loss on drying | ≤1.0% |
| Loss on ignition | ≤0.5% |
| Melting Point | ≥300℃ |
Packing & Storage
| Packing | In 25kg Fiber drum as per customer’s requirement |
| Storage | Store in dry,clean place ,avoid direct sun light. |
| Shipping | In room temperature |
Free Quote
At present, the company has more than 10 experienced export sales .
For samples, pricing, or more information, please call us at 0086-25-52397808 or mail to info@tolyltriazole-benzotriazole.com or . We will respond to you as soon as possible.
Tel: 0086-25-52397808
E-mail: info@tolyltriazole-benzotriazole.com


General Information
| Common Names | 3-Amino-5-mercapto-1,2,4-triazole | ||
| Structure | 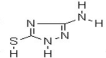 > > |
||
| CAS No. | 16691-43-3 | Boiling Point (℃) | 389.1±25.0 |
| Molecular Weight | 116.145 | Melting Point (℃) | ≥300 |
| Appearance | White crystalline powder | Density(g/cm3) | 1.7±0.1 |
| HS Code | 2933990090 | Flash Point (℃) | 189.1±23.2 |
| Safety Phrases | S26-S36-S36/37/39-S16 | ||
| RIDADR | F:Flammable;Xn:Harmful; | ||
| WGK Germany | 3 | ||
| Packaging Group | II; III | ||
| Hazard Class | 4.1 | ||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. Redness. Burning sensation. Itching. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Eyes | Redness. Pain. | Protective gloves. | Rinse opened eye for several minutes under running water. Then consult a doctor. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
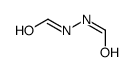
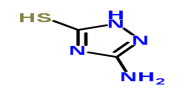
2,5-Dithiobiurea 3-Amino-5-mercapto-1,2,4-triazole
CAS NO.:142-46-1 CAS NO.:16691-43-3
Frequently Asked Questions
AMT Applications Explored
3-Amino-5-mercapto-1,2,4-triazole, also known as AMT or 3-amino-5-mercapto-1,2,4-triazole, is a chemical compound with the molecular formula C2H4N4S. It belongs to the class of compounds known as triazoles, which are heterocyclic compounds containing a five-membered ring with three nitrogen atoms and two carbon atoms. AMT contains an amino group (-NH2) and a thiol group (-SH), making it a versatile compound for various applications.
AMT has been used in several fields, including pharmaceuticals, agriculture, and materials science. Here are a few applications:
1. Pharmaceutical research: AMT has been studied for its potential medicinal properties. It has shown promise in inhibiting the activity of enzymes involved in certain diseases, such as metalloenzymes and carbonic anhydrase.
2. Agrochemicals: AMT has been investigated as a potential ingredient in pesticides and fungicides due to its ability to inhibit certain enzymatic processes in plants. It has shown efficacy against a variety of agricultural pests and diseases.
3. Corrosion inhibition: AMT has been used as a corrosion inhibitor in metalworking fluids and other industrial applications. Its thiol group can form a protective layer on metal surfaces, preventing or slowing down the corrosion process.
4. Coordination chemistry: AMT can act as a ligand in coordination complexes, forming coordination bonds with metal ions. These complexes have been studied for their catalytic and sensing properties.

